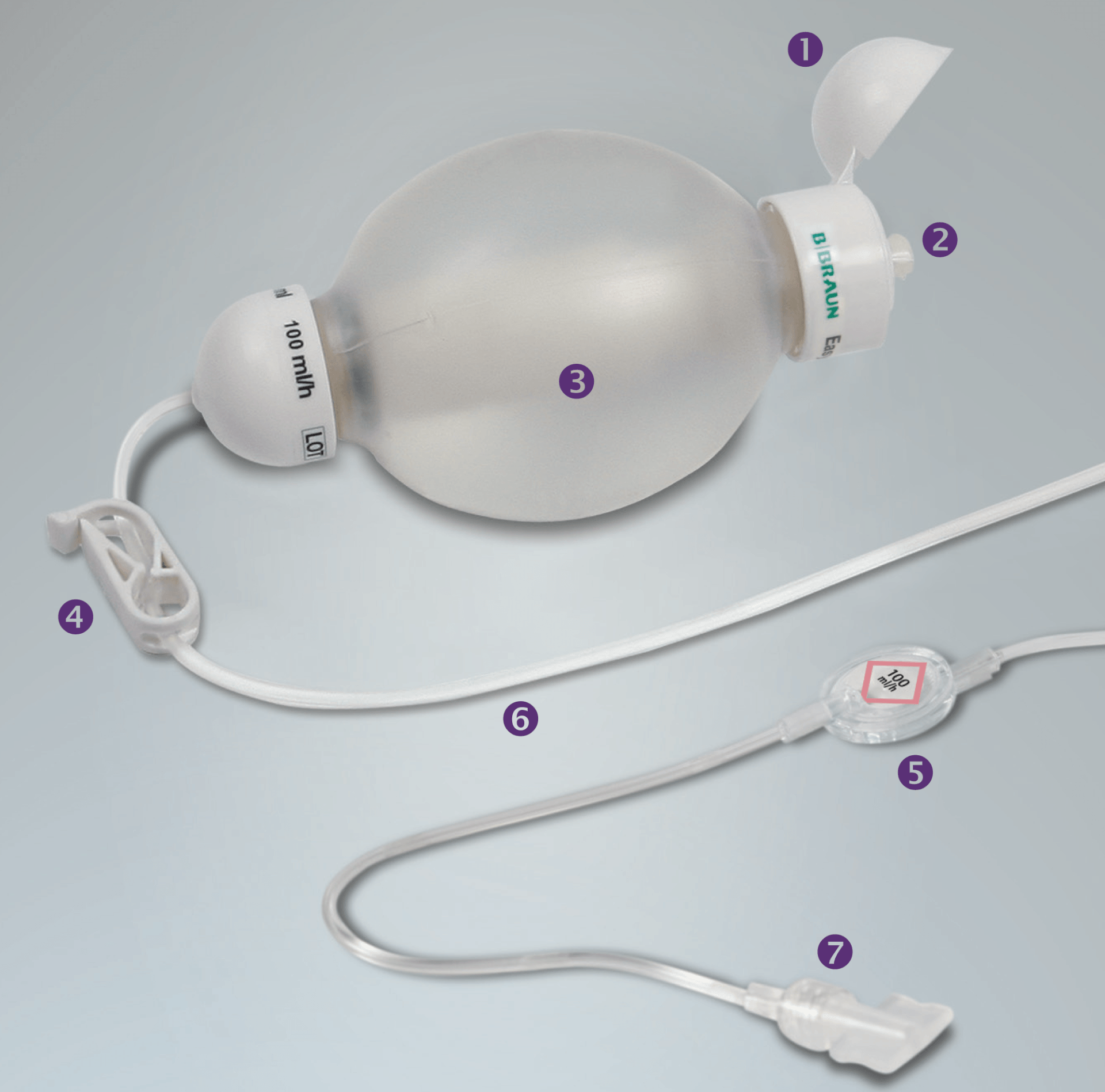
Lunch with Abbott SJM Team
SIMPLIFYING COMPLIANCE
We offer distinctive competencies leading technical teams through Design Control, and Product Realization to facilitate compliance and minimize development time. We provide subject matter expertise and guidance to the following processes:
- Guide R/D team through a simplified, gated, FDA/ISO/MDR compliant and performing process
- Speed up Design and Process FMECA
- Validate design significant parameters to their appropriate sigma levels
- Validate Process capability to designed sigma levels
- Validate existing test methods, and measurements processes
- Review and simplify quality procedures
- Qualify suppliers to meet the required capability, and establish vendor surveillance to ensure sustained capability
- Audit DHF, 510k/PMA/Technical File, Risk Management, and associated deliverables for communicability and compliance
- Coach management on how reducing variations reduces cost.
GUIDING FDA/ISO/EU MDR 2017
With over 20 years of experience implementing a closed loop feedback system for product lifecycle management, we have gained a profound knowledge of the regulatory trends, and will put your team ahead of the FDA/ISO/MDR compliance curve.
Lunch with Edwards'Team